
ART CONSERVATOR
DIGITAL
A PUBLICATION OF THE WILLIAMSTOWN + ATLANTA ART CONSERVATION CENTER
VOLUME 15. NO.2 THE 101 GUIDE to
disposable gloves
The 101 Guide to Disposable Gloves (and How to Find Out Which Ones Not to Use)
SALLY GUNHEE KIM | POSTGRADUATE FELLOW IN OBJECTS CONSERVATION
Before wearing gloves and masks in the public was a new normal, art conservators have often used disposable gloves in labs. Disposable gloves are worn because they offer dexterity, protection to the conservators, and to the objects being handled.
Even if disposable gloves offer the much-needed protection, they also give a false sense of security. It is important to note that there are various manufacturers that provide gloves. This means that, while the basic components of the gloves remain the same, different manufacturers source materials differently and use additives to give their products unique and desirable properties. Often, their recipes are proprietary and subject to change over time. As a common piece of protective equipment that is ensured for safety, their composition and potential degradation compounds are often overlooked and the chemicals used to produce them are unavailable and protected by a patent. Therefore, it is imperative to examine the gloves before use in conservation labs to determine if they provide the necessary protection without leaving unwanted residues on either the object or the conservator.

FIGURE 1. Seven disposable gloves in use at WACC.
This article will first discuss disposable gloves in general, outlining their chemical composition and associated properties. Then, the preparation for the Oddy test and FT-IR will be explained in steps. Finally, the results of gloves from different labs at WACC will be discussed in-depth. The goal of analysis was to determine which gloves should and should not be used in the labs.
While the focus of this article is on the analysis of the seven disposable gloves in use at WACC, the author hopes to share both the procedures and analytical results to help others in our professional field, our clients, and art institutions in choosing which gloves are appropriate for use in settings where artworks are frequently handled and to reproduce the Oddy tests that help inform such decisions.
CHEMICAL COMPOSITION of DISPOSABLE GLOVES
Monomers are Lego® block-like molecules of the same color and size. These building blocks, or monomers, often react with the identical molecules to form a larger polymer chain or network in a process called polymerization. These identical building blocks might construct to form a tree trunk or a part of a castle, perhaps more so, after watching the latest Netflix® special on Lego®. Like colorful Lego® blocks, monomers can react with different monomers to form a new combination, for example, nitrile gloves, the details of which will be expanded further below.
Vinyl Gloves
While vinyl gloves have their benefits, their chemical composition is unstable. Polyvinyl chloride is very sensitive to elevated temperatures and light. Upon exposure, polyvinyl chloride will undergo crosslinking and photo-oxidation, leading to discoloration and loss in desired mechanical properties; for example, they lose elasticity. This means that the gloves degrade quickly, producing undesirable residues on their surfaces that will be deposited on both artifacts and the hands of the conservator: hydrogen chloride, aromatic and aliphatic hydrocarbons, and non-condensable gases [4].
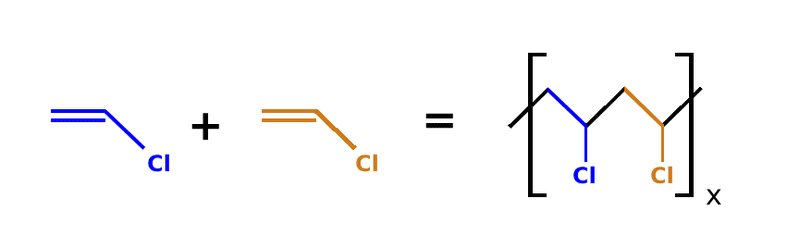
FIGURE 2 . Reaction between polyvinyl chloride monomers.
Nitrile Gloves
Currently, nitrile gloves hold the largest market in the disposable gloves sector for having the lowest material cost yet providing positive mechanical and chemical performance qualities. They are a good choice for a wide spectrum of chemicals. Additionally, unlike polyvinyl chloride, nitrile is chemically stable; nitrile will not degrade or discolor immediately, hence, not leaving residues on the surfaces when handling.
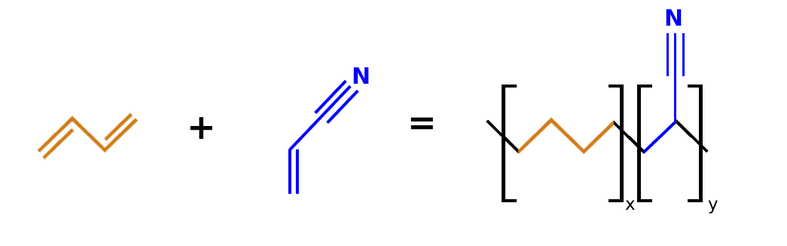
FIGURE 3 . Reaction between acrylonitrile butadiene (left) and acrylonitrile (right)
DIFFERENT COMPANIES, DIFFERENT RECIPES, HENCE DIFFERENT PROPERTIES
Even within the same manufacturer, there could be changes in their recipes with ingredients sourced and processed from different locations at different times. This can be distinguished by serial numbers on boxes. Outlined below are two examples of gloves’ common properties:
Powder-Free
Even though the nitrile gloves may have been washed rigorously to remove residues from chlorination, it is recommended not to use chlorinated gloves when handling artworks. Chlorine-based chemicals may release volatile organic compounds (VOCs) as well as other toxic substances that cause metals, in particular silver and copper, to corrode [5].
Accelerators are chemicals that act as catalysts to increase the speed and lower the temperature at which gloves are produced. These components also lend increased elasticity, strength and integrity. Despite many advantages of accelerators, their addition is a concern for many conservators, in addition to potential allergic reactions.
Accelerator-free
Accelerators are typically sulfur-based to facilitate the vulcanization process that is common to natural rubber gloves or synthetic ones, such as nitrile. Vulcanization is a manufacturing process during which synthetic materials are heated with sulphur and accelerator at a high temperature to form cross-links between polymers to form the final product. The sulfur component may react and tarnish metals, in particular, silver. Thankfully, there is an alternative: just like how some foods are processed in nut-free facilities, accelerator-free gloves are manufactured without sulfur-based chemicals. In addition to minimizing potential damage to silver and other metallic artifacts, these gloves have a lower risk of irritating skin and can be more durable and reliable than common nitrile or vinyl gloves.
ODDY TESTING
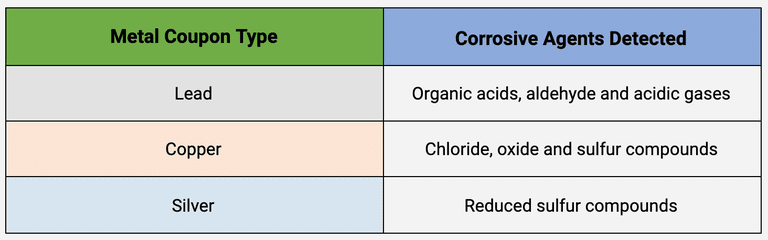
TABLE 1 . The corrosive agents detected by each metal coupon during an Oddy Test in response to unstable materials.
The seven gloves in question are manufactured from five different companies and have been in use in six departments at WACC (fig.1). To respect the confidentiality of the manufacturers, their brand names and serial numbers were not included in the results and analysis. Instead, each manufacturer’s name is alphabetically ordered from A to E, and their products numerically organized accordingly (table 2). The procedure to preparing the Oddy tests are described in steps below.
The Oddy Test Procedure
1. Obtain a sample of the test material (e.g., a sample from a nitrile glove) and place it in a clean glass container (e.g., PYREX® storage bottle). All glassware should have already been thoroughly cleaned with lab glassware cleaning detergent, rinsed with deionized water several times, and dried before use. It is recommended that the size ratio of the sample to the container is at least 1:5 (weight/volume); for example, use 2g of test material for a 10mL container. Each container should have a silicone stopper.
2. Place a smaller glass vial or test tube stuffed with cotton wool that has been dampened with deionized water. This will help ensure that high humidity is maintained in the container.
3. Insert a relative humidity moisture indicator paper so that the relative humidity inside the glass container at a relative humidity of 100% can be monitored after it is sealed.
4. Prepare the metal coupons: cut a 1”-long by ½”-wide piece from each metal sheet (e.g., lead, copper and silver). It is preferred if each metal is at least 99% pure . Use glass bristle brushes to remove surface oxidation and dirt from the coupons’ surfaces. When using the brushes, it is strongly recommended to wear a respirator mask and to keep a vacuum cleaner nearby to avoid breathing in abrasive powders. Each metal should be cleaned with one brush at a time to avoid cross contamination.
5. Make three incisions in the silicon stopper that is used to seal the container. The metal coupons should be pushed into the incisions so they can be inserted vertically into the container without touching each other (fig. 4). It is important that metals do not touch each other or the sample being analyzed.
6. Seal the container with the silicon stopper.
7. Repeat steps 1 – 6 with other test samples.
8. Set-up the control. For each set of Oddy test samples, one control test without sample material must be carried out at the same time so that the effects of temperature and relative humidity on the metal coupons alone can be used as a reference for comparison. For further clarification, the control is an experiment where all factors are held constant except for the independent variable, which is the sample of the material being tested. In the case of an Oddy test, the control test is prepared with the glass container, a silicon stopper, metal coupons, relative humidity moisture indicator and everything else, except the test material (e.g., nitrile or vinyl glove) itself.
9. Place the prepared, sealed containers inside of an oven at 80°C (176°F) (fig.5).
10. Leave the containers in the oven for five days, and check the container occasionally to check its temperature and relative humidity. While five days were used at WACC, samples can be left in their containers for up to 28 days.
11. After five days, remove containers from the oven and allow them to cool to room temperature.
12. Remove metal coupons from each container with tweezers. Observe the level of corrosion and compare coupons from the different samples (fig. 6).

FIGURE 4. Prepared metal coupons inserted into the slits made in the silicon stopper

FIGURE 5. Prepared, sealed containers with test materials in the oven
Results
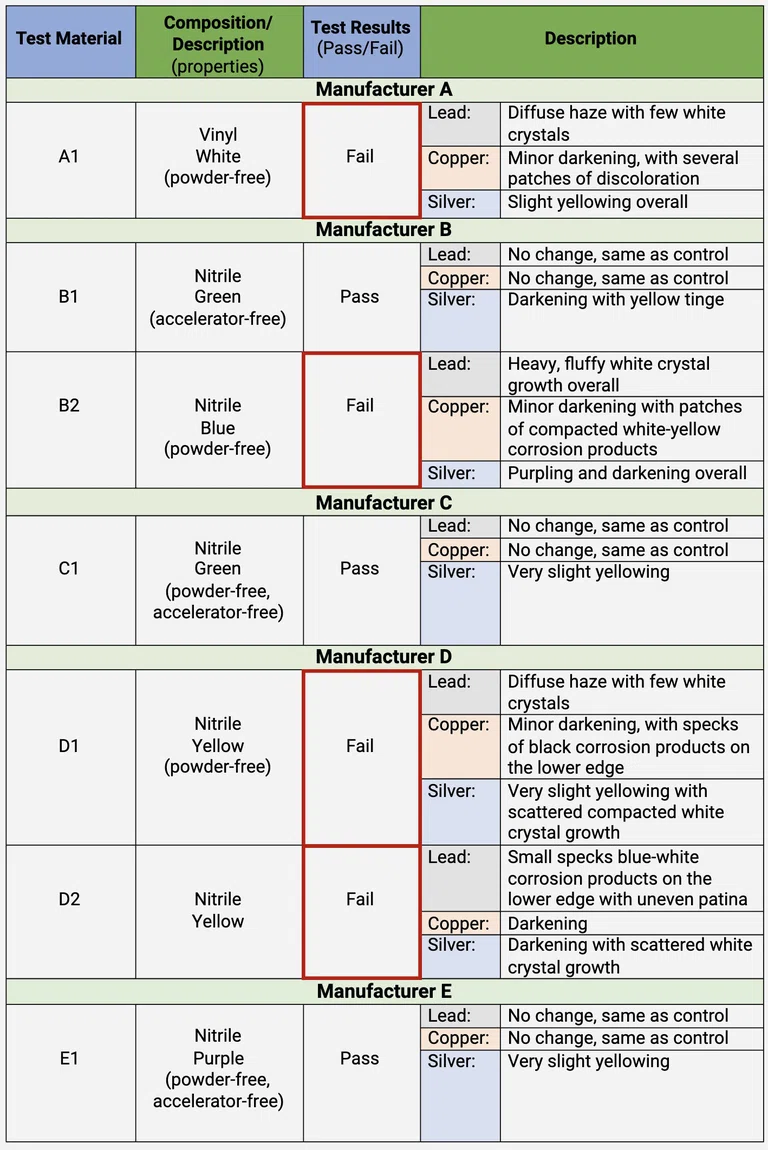
TABLE 2 . The results of the seven disposable gloves from the Oddy test.
Discussion | Preliminary conclusions based on the Oddy Tests
Another observation in the Oddy tests is that, for the powder-free nitrile and vinyl gloves, all lead coupons exhibited signs of homogeneous diffuse haze and white crystal growth, and all copper coupons showed signs of darkening. There are two possibilities to explain the observation:
1. The gloves may have been treated with chlorination. Even if manufacturers state that chlorinated gloves are rinsed vigorously, residues may have remained on the gloves and released VOCs that caused lead and copper coupons to corrode in the Oddy test [7].
2. A polymer slip coating may have been applied on the interior of some powder-free gloves. The unspecified polymer slip coating may contain chemicals that off-gas VOCs: silicone polymer, polyurethane, and polymer-blends [8]. Silicone-based materials are relatively inert and stable, so they are widely used in conservation labs. However, similar to nitrile gloves, they may have additives that may selectively cause metal to corrode, which in turn, attack on the silicone polymers, leading to embrittlement and loss in their mechanical strength. VOCs may leach out of silicone-based conservation materials over time, when exposed to oils [9].
While it is concerning that more than half of the gloves failed the Oddy tests, it is important to keep in mind that the tests are not failsafe for three reasons:
1. Surface preparation of the metal sheets vary, thus affecting results of corrosion [10]. Also, corrosion observed on each metal coupons are hardly assessable on the visual inspections alone when there has already been a mixture of off-gassed contaminants in the glass container.
2. Sampling, preparation and storage of the materials to be tested in laboratories can significantly affect the Oddy test results [11]. For example, the materials may have already absorbed contaminants already present in the air.
3. Products such as proprietary gloves change over time so there is no guarantee that results related to the product will remain consistent. The accelerator-free gloves that passed Oddy tests at the time of examination may fail in the future with the changes in recipes and processes by the manufacturer.
Overall, the Oddy test demonstrated that powder-free nitrile and vinyl gloves should no longer be used in the conservation labs at WACC. However, the Oddy tests did not specifically identify the pollutants that caused corrosion in the metal coupons; organic analysis is necessary for their identification.
Further Discussion | Gloves that Caused the Worst Corrosion Reactions in the Oddy Test
Safety Data Sheets (SDS) may identify the components in a material and provide the associated health and safety hazard information. The SDS for the ‘B2 gloves’ is unavailable because a SDS is not required by law under Hazard Communication Standard section 29 CRF 1910.1200 when chemical components in the final product do not present a health and safety hazard to the wearer. Manufacturers may also do this to protect their “secret recipe” from competitors in their industry. This meant that ingredients in the disposable gloves were not readily available for interpretation on what caused corrosion.
Thus, Fourier-transform infrared spectroscopy (FT-IR) was used to identify the pollutant in the disposable gloves that possibly caused corrosion in the metal coupons for the ‘B2 gloves’. Analyzing the additives in this pair of gloves can be used to supplement the observations from Oddy tests and to specifically justify why it cannot be used in the conservation lab.
FT-IR Analyses
Procedure
The finger was immersed in the glass tube for two different periods of two and seven days to observe if the obtained solution will have a higher concentration of the extracted additives over time and if traces of other additives could also be extracted. The solution was dried into a film on a microscope slide (fig.8). The film was then sampled for identification with FT-IR [12].

FIGURE 7 . Extracting additives from the gloves with varying solvents.

FIGURE 8 . Drying extracted additives as a film for analysis with FT-IR.
Results
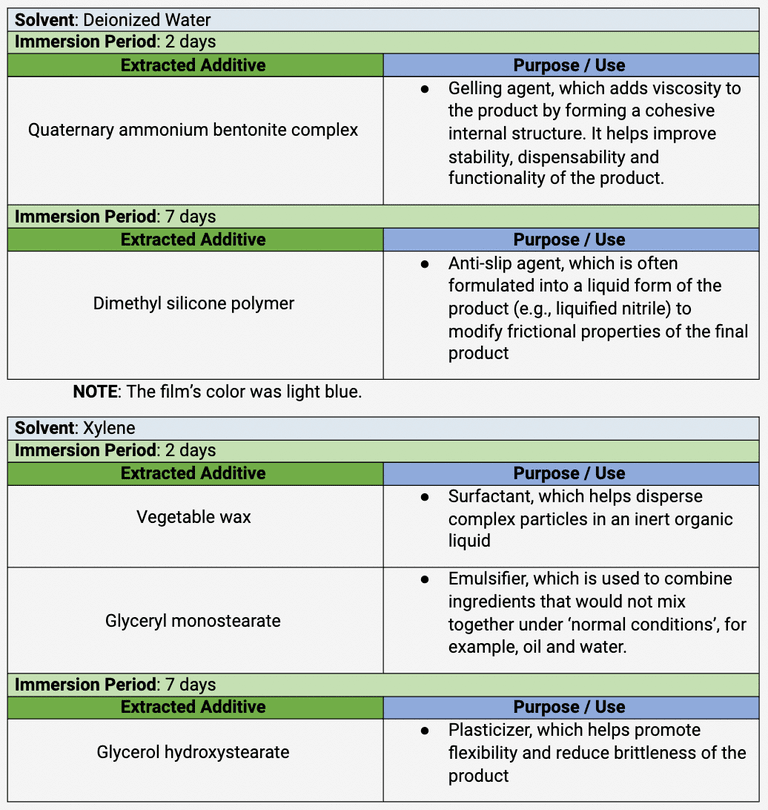
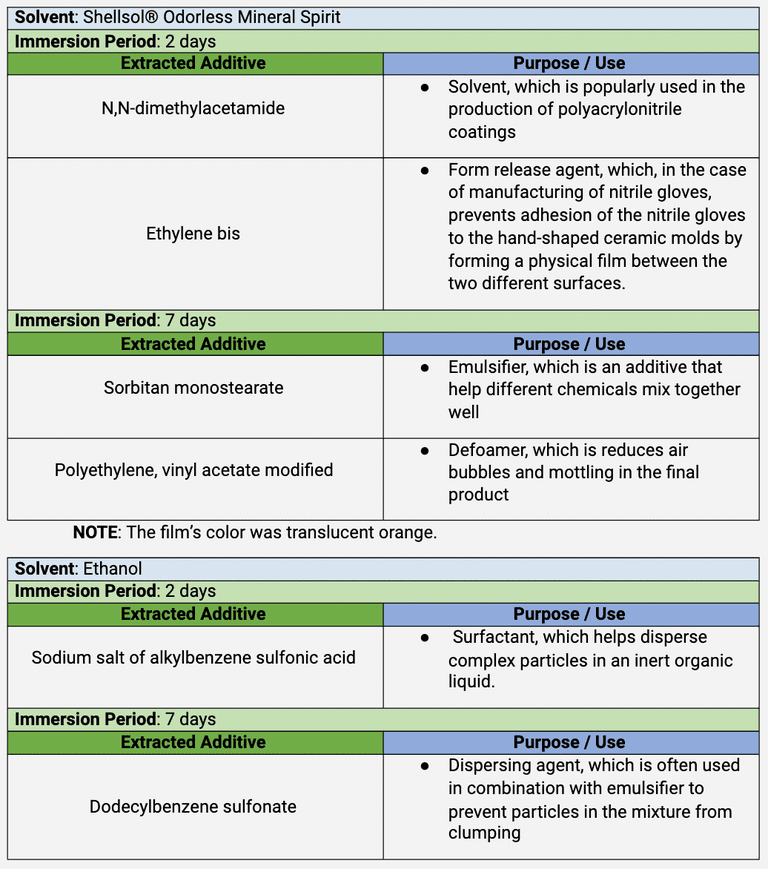
Table 3 . The identification of additives in the ‘B2 gloves’
Discussion | Preliminary conclusions based on further analysis with FT-IR
Lead stearate has an appearance of powdery white solid, similar to those observed on the coupon. Metallic stearate powders (e.g., zinc stearate) are commonly used in the manufacture of powder-free nitrile gloves to substitute for cornstarch. They are added to gloves during chlorination to not only improve the donning and removal but also slow down the degradation of the gloves [13]. To clarify, lead stearate is not used in the gloves; rather, zinc stearate in the gloves reacted with the lead coupons in the Oddy test, leading to the formation of lead stearate. The gloves on their own do not contain lead; otherwise, a SDS would have been provided to give information on chemical components in the product that may present a health and safety hazard to the wearer.
In addition to stearate, the corroded lead coupon associated with the ‘B2 gloves’ likely detected an acidified form of stearate: stearic acid, which is an organic acid. Stearic acid is also commonly used in the manufacture of plastics as a lubricant during injection molding and as a mold release for rubber baked on ceramic molds. Nitrile gloves are produced by dipping hand-shaped ceramic molds in tanks filled with liquified nitrile, which are then heated at a high temperature to form the gloves as they dry [14]; this is known as vulcanization. Nitrile gloves are hard to strip from the molds, so it is possible that in the manufacture of B2 gloves, stearic acid was added as a mold release agent. Some stearic acid might have remained in the final product and reacted with the lead coupon to form corrosion products identified as lead stearate.
CONCLUSION
As previously discussed, the Oddy test is only useful for detecting whether or not VOCs are off-gassed from materials under certain experimental conditions. Comparison of changes in the metal coupons is subjective, so judgements of “Pass or Fail” should not be regarded as reproducible, infallible scientific observations of the tested materials [15]. Still, despite its lack of precision, the Oddy test has been widely used in museum contexts for its inexpensiveness and ease of result interpretation [16]. For products that failed the Oddy tests, SDS sheets could be used to reference chemicals that may lead to corrosion in artworks. In cases where the SDS sheet is not provided, for example, the ‘B2 gloves’, FT-IR can be used to identify the culprit of corrosion observed in metal coupons from the Oddy tests.
FT-IR indicated the possible presence of stearate or stearic acid in the lead coupon pertaining to sample B2, which displayed the worst VOC products. Lead coupons detect organic acids, aldehyde, and acidic gases off-gassed from the test materials. Because monostearate was detected in the films extracted from the ‘B2 gloves’ with both xylene and Shellsol® OMS and also in the corrosion products, it is assumed that the corrosion product is lead stearate. However, it is still too early at this stage to conclude that sorbitan monostearate and glyceryl monostearate were the only two additives that caused corrosion reactions. For example, another additive extracted with Shellsol® OMS is ethylene bis, which is derived from the reaction of ethylenediamine and stearic acid.
The combined use of Oddy tests and FT-IR demonstrates that while disposable gloves help provide protection in conservation labs, they also give a false sense of security due to variable corrosion-causing additives. It is imperative to examine any brand or type of gloves before use in conservation labs since results demonstrate that additives may vary not only between manufacturers but also between two different types of gloves made by the same manufacturer; for example, the ‘B1 gloves’ from ‘manufacturer B’ passed the Oddy test, but the ‘B2 gloves’ did not and caused the worst corrosion in the metal coupons.
The Oddy test was the preferred method for detection of VOCs in this experiment because it is the easiest and most cost-efficient way to determine the best gloves for use in conservation labs. It does not need to be limited to disposable gloves, and can be applied to a range of materials that come into contact with your collection. Where the resources are limited or unavailable, Williamstown Art Conservation Center (WACC) can provide Oddy test services to interested clients. Similarly, corrosion samples from the metal coupons may be sent to WACC for identification with FT-IR. Choosing the most stable materials for use in contact with artworks is an invaluable investment for the long-term preservation of cultural heritage objects.
Acknowledgement
I would like to express my special thanks of gratitude to Hélène Woodard-Gillette for encouraging me to research on the topic of disposable gloves used at WACC. I would also like to thank Christine Puza for her help in obtaining liquid nitrogen needed for data collection with FT-IR and Matthew Hamilton for his photography.
Photography Credit
REFERENCES
[1] Strickler, Laura, Stephanie Gosk, Lisa Cavazuti, and Brenda Breslauer. “Trump Admin Is 'Woefully behind' in Stockpiling Medical Gloves as Covid-19 Surges.” In NBC News: Coronavirus, October 29, 2020. https://www.nbcnews.com/politics/politics-news/trump-admin-woefully-behind-stockpiling-medical-gloves-covid-19-surges-n1245298.
[2] Magee, Steven. “Environmental Radiation Researcher.” In EnvironmentEMR (tweet), n.d. https://twitter.com/EnvironmentEMR
[3] PennEHRS. “Fact Sheet: Disposable Nitrile Gloves in Chemical Labs.” In University of Pennsylvania Environmental Health & Radiation Safety, December 2018. https://ehrs.upenn.edu/health-safety/lab-safety/chemical-hygiene-plan/fact-sheets/fact-sheet-disposable-nitrile-gloves.
[4] McNeill, Ian C., Livia Memetea, and William J. Cole. “A Study of the Products of PVC Thermal Degradation.” In Polymer Degradation and Stability 49, no. 1 (1995): p.181–91. https://doi.org/10.1016/0141-3910(95)00064-s.
[5] Jeffers, Will. “Nitrile Gloves.” In ConsDistList, June 26, 2003. https://cool.culturalheritage.org/byform/mailing-lists/cdl/2003/0873.html.
[6] Banik, Gerhard. “The Oddy Test – What Works and What Doesn’t.” In KLUG Conservation (commentary), June 3, 2013. https://www.klug-conservation.com/The-Oddy-Test-What-Works-and-What-Doesn-t.
[7] Ziegler, Julia, Charlotte Kuhn-Wawrzinek, Margarete Eska, and Gerhard Eggert. “Popping Stoppers, Crumbling Coupons - Oddy Testing of Common Cellulose Nitrate Ceramic Adhesives.” In ICOM-CC 17th Triennial Conference: Glass and Ceramics, 2014.
[8] Hourglass International, Inc. “Powder-Free Exam Glove Choices - Chlorinated vs. Polymer-Coated.” In Hourglass International
Monitoring for Gaseous Pollutants in Museum Environments (post), May 18, 2015. https://hourglass-intl.com/powder-free-exam-glove-choices-chlorinated-vs-polymer-coated/.
[9] Klostermeyer, Stephen H. “Controlling VOC Emissions from Silicone-Based Coatings.” In ASI: Adhesives & Sealants Industry (case study), April 23, 2020. https://www.adhesivesmag.com/articles/97714-controlling-voc-emissions-from-silicone-based-coatings.
[10] Banik, Gerhard. “The Oddy Test – What Works and What Doesn’t.” In KLUG Conservation (commentary), June 3, 2013. https://www.klug-conservation.com/The-Oddy-Test-What-Works-and-What-Doesn-t.
[11] Ibid.
[12] The spectrometer used at WACC is Thermo Scientific™ Nicolet™ iS50 FT-IR.
[13] Canosa, Elyse, Sara Norrehed, Marei Hacke, and Anna Wiman. “Characterization of Emissions from Display Case Materials.” In Swedish National Heritage Board, June 2019. https://doi.org/10.13140/RG.2.2.22913.71524.
[14] Omni Internationa Corp. “How Nitrile and Vinyl Gloves are Made.” In Nitrile Gloves (blog), n.d. https://omnigloves.com/2020/07/17/how-nitrile-and-vinyl-gloves-are-made/.
[15] Banik, Gerhard. “The Oddy Test – What Works and What Doesn’t.” In KLUG Conservation (commentary), June 3, 2013. https://www.klug-conservation.com/The-Oddy-Test-What-Works-and-What-Doesn-t.
[16] Grzywacz, Cecily M. Los Angeles, CA: The Getty Conservation Institute, 2006.


